上海交大方景源团队:具核梭杆菌是如何诱发大肠癌的?在肠癌的诊断和治疗中有哪些应用价值? |
 |
基于具核梭杆菌在结直肠癌发生发展中的重要作用,具核梭杆菌被认为是结直肠癌的治疗靶点之一。甲硝唑能清除异种移植CRC小鼠体内的具核梭杆菌,有效缩小肿瘤体积。非甾体抗炎药阿司匹林也能消除具核梭杆菌,其对结直肠癌的预防作用可能部分通过此介导。
既然抗生素有了,那么比抗生素更能针对单一细菌的噬菌体也可能有了。研究表明,携带伊立替康纳米颗粒的噬菌体可以靶向并定植于梭杆菌属的肿瘤,并调节肿瘤的生长[33]。
发现衍生自FadA和E-钙粘蛋白结合位点的11-aa抑制肽能够防止具核梭杆菌在肿瘤中定居并与CRC细胞结合[7]。类似的药物还包括抗FadA单克隆抗体,但是否能降低CRC的发病率还是未知数。这种基于细菌毒力因子的治疗需要更多的临床证据。
此外,粪便细菌移植,以及饮食、益生菌、益生元和后生动物等可以调节肠道微生物组成的方法,理论上具有辅助治疗CRC的潜力,但还需要更多更大规模的研究来验证。
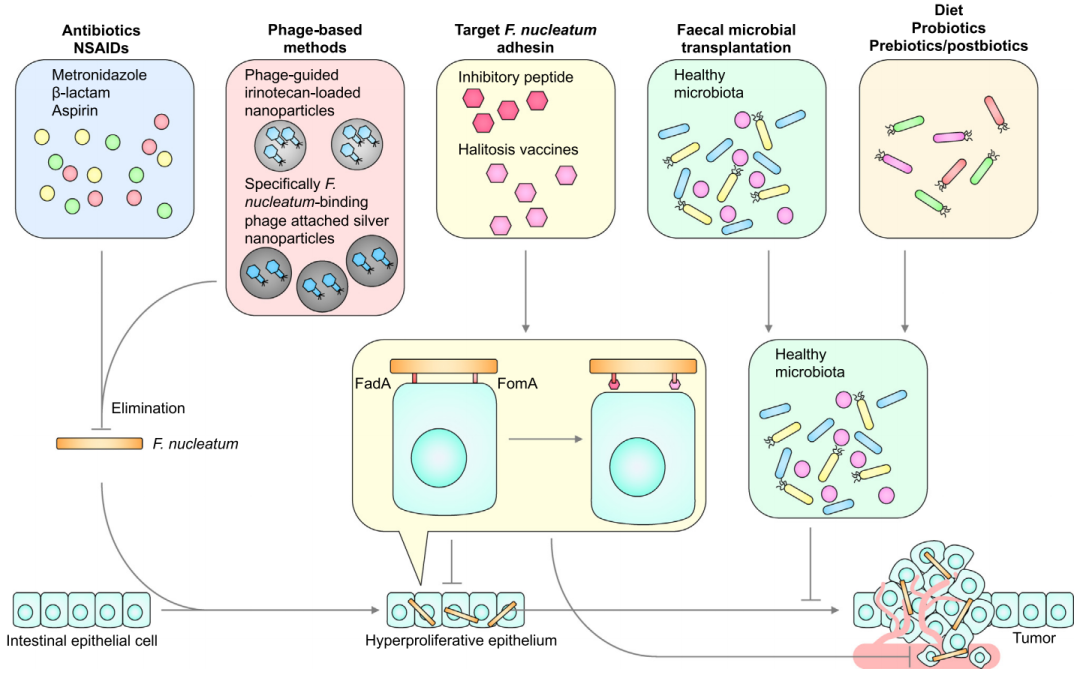
靶向具核梭杆菌的潜在治疗方法
具核梭杆菌是一种与癌症相关的肠道微生物,目前已被广泛研究,无论是致病机制还是作为生物标志物和治疗靶点的潜力。
但是,我们也应该认识到,肠道微生物之间存在复杂的相互作用,可能会干扰具核梭杆菌与结直肠癌的关系。同时,考虑到肿瘤的特异性和患者的个体差异,具核梭杆菌靶向治疗的效果还有很多未知数。就像单纯对肿瘤和肠道微生物学的研究一样,在这个交叉领域,我们还有很长的路要走。
参考资料:
[1] Sung H,Ferlay J,Siegel R L,等.全球癌症统计2020:全球185个国家36种癌症的发病率和死亡率估计[J].CA:临床医生癌症杂志,2021,71(3): 209-249。
[2] Siegel R L,等.癌症统计,2022[J].CA:临床医生癌症杂志,2022,72: 7-33。
[3]寇克欧,中津克,戴,等.肠道真菌菌群失调与结直肠癌的生态改变[J].Gut,2019,68(4): 654-662。
[4]柯思迪,盖弗斯,佩达马鲁,等.基因组分析鉴定梭杆菌与结直肠癌的关系[J].中国医学,2002 .基因组研究,2012,22(2): 292-298。
[5] Castellarin M,Warren R L,Freeman J D,等.具核梭杆菌感染在人类结直肠癌中普遍存在[J].基因组研究,2012,22(2): 299-306。
[6]李,李.口腔微生物群落的动态变化及其与宿主的相互作用[J].自然评论微生物学,2018,16(12): 745-759。
[7] Rubinstein M R,Wang X,Liu W,等.具核梭杆菌通过其FadA粘附素调节E-cadherin/-catenin信号通路促进结直肠癌发生[J].细胞宿主微生物,2013,14(2): 195-206。
[8]于廷春,郭芳,于燕,等.具核梭杆菌通过调节自噬促进大肠癌耐药[J].细胞
, 2017, 170(3): 548-563. e16.[9] Wang N, Fang J Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer[J]. Trends in Microbiology, 2022.
[10] Kostic A D, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment[J]. Cell host microbe, 2013, 14(2): 207-215.
[11] Hong J, Guo F, Lu S Y, et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer[J]. Gut, 2021, 70(11): 2123-2137.
[12] Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor B, and up-regulating expression of microRNA-21[J]. Gastroenterology, 2017, 152(4): 851-866. e24.
[13] Zheng X, Liu R, Zhou C, et al. ANGPTL4-mediated promotion of glycolysis facilitates the colonization of fusobacterium nucleatum in colorectal cancer[J]. Cancer Res, 2021, 81(24): 6157-6170.
[14] Abed J, Emg rd J E M, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc[J]. Cell host microbe, 2016, 20(2): 215-225.
[15] Casasanta M A, Yoo C C, Udayasuryan B, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration[J]. Science signaling, 2020, 13(641): eaba9157.
[16] Hu L, Liu Y, Kong X, et al. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF- B/S100A9 Cascade[J]. Frontiers in immunology, 2021, 12: 658681.
[17] Chen T, Li Q, Wu J, et al. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism[J]. Cancer Immunology, Immunotherapy, 2018, 67(10): 1635-1646.
[18] Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack[J]. Immunity, 2015, 42(2): 344-355.
[19] Kaplan C W, Lux R, Huynh T, et al. Fusobacterium nucleatum apoptosis-inducing outer membrane protein[J]. Journal of dental research, 2005, 84(8): 700-704.
[20] Kaplan C W, Ma X, Paranjpe A, et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes[J]. Infection and immunity, 2010, 78(11): 4773-4778.
[21] Komiya Y, Shimomura Y, Higurashi T, et al. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity[J]. Gut, 2019, 68(7): 1335-1337.
[22] Mima K, Nishihara R, Qian Z R, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis[J]. Gut, 2016, 65(12): 1973-1980.
[23] Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma[J]. Cancer research, 2014, 74(5): 1311-1318.
[24] Tahara T, Yamamoto E, Madireddi P, et al. Colorectal carcinomas with CpG island methylator phenotype 1 frequently contain mutations in chromatin regulators[J]. Gastroenterology, 2014, 146(2): 530-538. e5.
[25] Saito K, Koido S, Odamaki T, et al. Metagenomic analyses of the gut microbiota associated with colorectal adenoma[J]. PLoS One, 2019, 14(2): e0212406.
[26] Park H E, Kim J H, Cho N Y, et al. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma[J]. Virchows Archiv, 2017, 471(3): 329-336.
[27] Kong C, Yan X, Zhu Y, et al. Fusobacterium Nucleatum Promotes the Development of Colorectal Cancer by Activating a Cytochrome P450/Epoxyoctadecenoic Acid Axis via TLR4/Keap1/NRF2 SignalingFn Promotes Colorectal Cancer by CYP2J2/12, 13 EpOME Axis[J]. Cancer Research, 2021, 81(17): 4485-4498.
[28] Chen S, Su T, Zhang Y, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7[J]. Gut microbes, 2020, 11(3): 511-525.
[29] Zhang S, Yang Y, Weng W, et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer[J]. Journal of Experimental Clinical Cancer Research, 2019, 38(1): 1-13.
[30]Huang Q, Peng Y, Xie F. Fecal fusobacterium nucleatum for detecting colorectal cancer: a systematic review and meta-analysis[J]. The International journal of biological markers, 2018, 33(4): 345-352.
[31]Liang Q, Chiu J, Chen Y, et al. Fecal Bacteria Act as Novel Biomarkers for Noninvasive Diagnosis of Colorectal CancerFecal Bacterial Markers for Colorectal Cancer[J]. Clinical Cancer Research, 2017, 23(8): 2061-2070.
[32]Kurt M, Yumuk Z. Diagnostic accuracy of Fusobacterium nucleatum IgA and IgG ELISA test in colorectal cancer[J]. Scientific reports, 2021, 11(1): 1-6.
[33]Zheng D W, Dong X, Pan P, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy[J]. Nature biomedical engineering, 2019, 3(9): 717-728.
医药网新闻
- 相关报道
-
- PNAS:免疫疗法新突破!NOD2基因变异助力癌症治疗 (2025-08-06)
- 《自然· 医学》:按照膳食指南吃可能还不够!最新临床试验显示,即使遵照指南要求吃饭,多吃超加工食品仍会阻碍减重 (2025-08-06)
- 施一公团队发表最新PNAS论文 (2025-08-06)
- 致癌代谢物2HG竟是肥胖“隐形推手”?!Nat Metabol 最新研究中科学家揭秘肥胖发生新机制 (2025-08-05)
- Sci Adv:科学家有望重新训练中性粒细胞来靶向治疗乳腺癌 (2025-08-05)
- 特洛伊木马:复旦大学最新论文登上Cell子刊封面 (2025-08-05)
- TN:南京医科大学团队发现,长期运动可通过改善脑膜淋巴管结构和引流功能,减轻AD病理,并改善小鼠认知功能 (2025-08-05)
- 柳叶刀:塑料危机——对人类从摇篮到坟墓的健康威胁 (2025-08-05)
- Nature子刊:中山大学林浩添/陈崴团队开发AI模型,利用视网膜图像无创诊断慢性肾病 (2025-08-05)
- 四部分脱手!医疗科普不是“流量生意” (2025-08-05)
- 视频新闻
-
- 图片新闻
-
医药网免责声明:
- 本公司对医药网上刊登之所有信息不声明或保证其内容之正确性或可靠性;您于此接受并承认信赖任何信息所生之风险应自行承担。本公司,有权但无此义务,改善或更正所刊登信息任何部分之错误或疏失。
- 凡本网注明"来源:XXX(非医药网)"的作品,均转载自其它媒体,转载目的在于传递更多信息,并不代表本网赞同其观点和对其真实性负责。本网转载其他媒体之稿件,意在为公众提供免费服务。如稿件版权单位或个人不想在本网发布,可与本网联系,本网视情况可立即将其撤除。联系QQ:896150040






















